the Harris Lab
Research Overview
The Genetics of Tissue Regeneration
Most human tissues damaged by injury or disease have limited capacity to regenerate, and current treatments rarely restore full function. In contrast, many other species can regrow complex structures, even entire organs that lack regenerative ability in humans. Our lab investigates the genetic and molecular mechanisms that enable this remarkable regenerative capacity. We aim to identify the key factors that drive tissue regrowth and to understand how their activity is regulated throughout the regeneration process. By defining these fundamental principles, we seek to lay the groundwork for new strategies to enhance tissue repair and promote regeneration in humans.
o study these mechanisms, we use the powerful genetic model organism Drosophila melanogaster (the common fruit fly). The larval organs of Drosophila, known as imaginal discs, can regenerate following many types of damage, including physical injury, irradiation, and genetically induced cell loss.
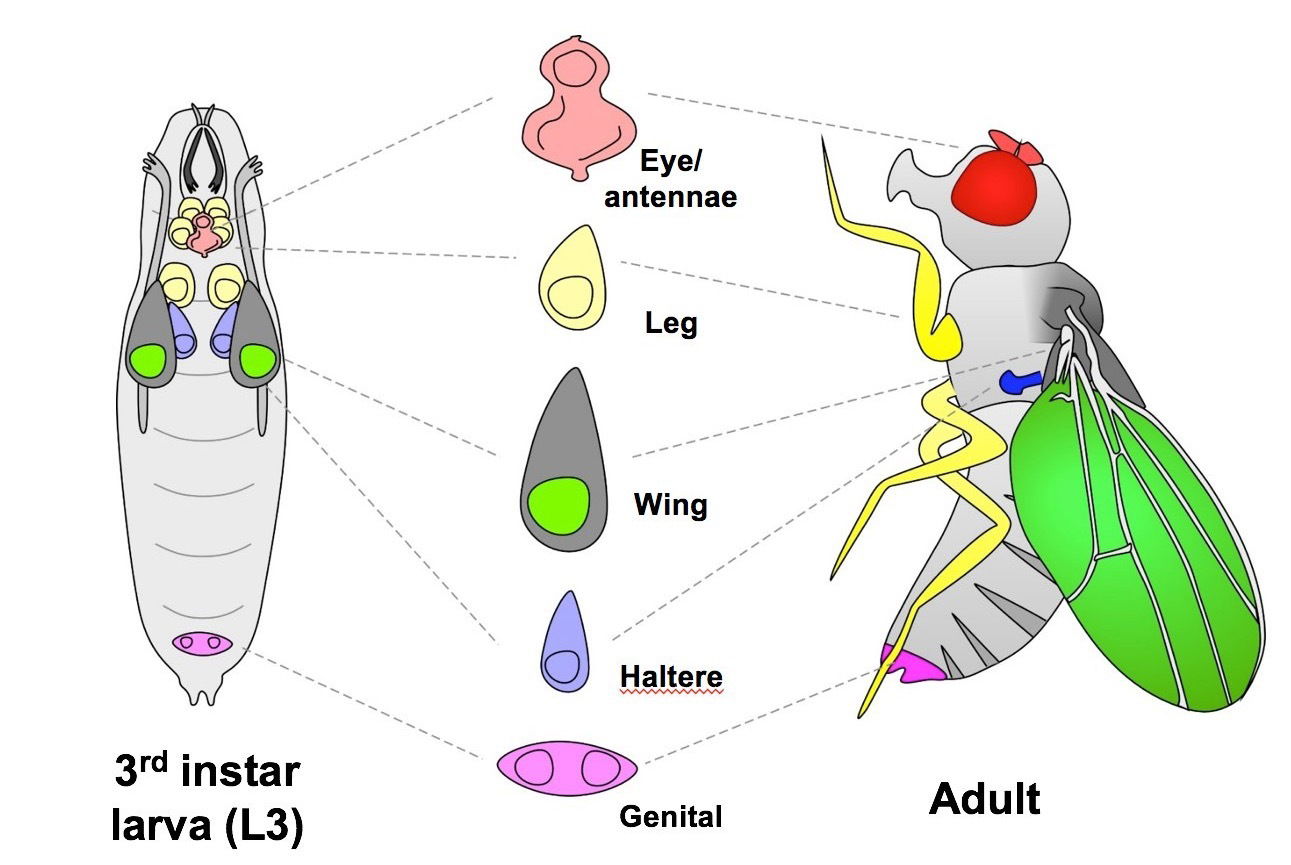
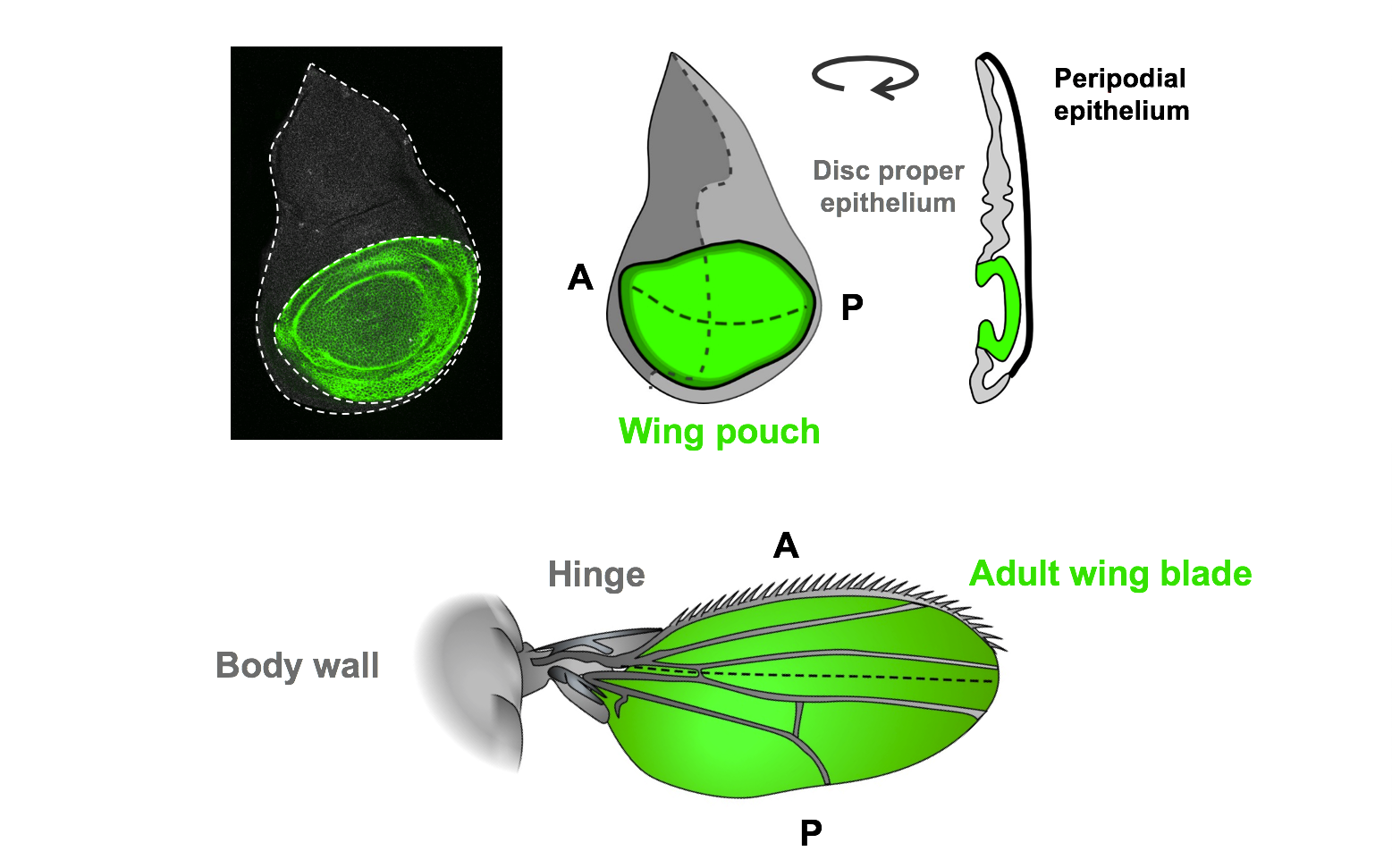
Among these tissues, the wing imaginal disc—an epithelial structure that forms the adult wing—has become a classic model for studying tissue growth and regeneration. Because the genetic pathways controlling regeneration are highly conserved between Drosophila and mammals, insights gained from this system are broadly relevant to understanding and promoting regeneration in human tissues.
Genetically inducing tissue damage and stimulating regeneration
To investigate how tissues regenerate, our lab developed DUAL Control, a versatile genetic system that enables precise induction of tissue damage and independent manipulation of gene expression during regeneration in Drosophila. Traditional ablation systems rely on GAL4/UAS-driven apoptosis, which restricts the simultaneous use of other GAL4- or UAS-based tools commonly used in fly genetics. DUAL Control overcomes this limitation by employing a split LexA system, allowing ablation to be spatially and temporally controlled, independent of GAL4 activity.
In this system, one component (LexA:DBD) is driven by a tissue-specific spalt (salm) enhancer, while the other component (p65) is expressed under a heat-shock promoter. A brief heat shock induces p65 expression, transiently reconstituting LexA activity only in the distal wing pouch. This drives expression of any lexAop-transgene, including those that trigger apoptosis (via JNK-dependent or -independent pathways) or necrosis (through leaky ion channel induction).
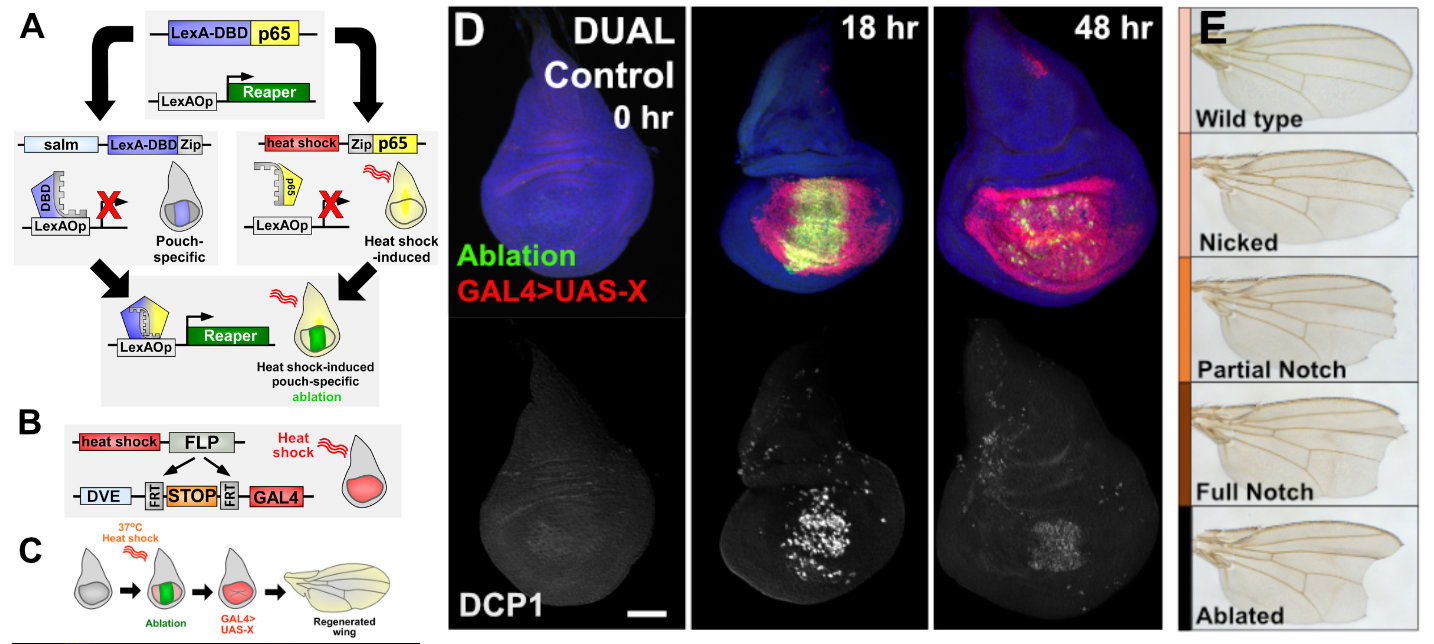
To manipulate genes in regenerating cells, we combine DUAL Control with DVE>>GAL4, a heat shock–activated driver expressed in the cells surrounding the injury that contribute to new tissue growth. A single heat shock therefore induces both targeted ablation and UAS-driven transgene expression in regenerating cells, enabling functional analysis of virtually any gene during tissue repair. Regenerative outcomes can then be assessed by immunofluorescent imaging of imaginal discs at defined time points or by quantifying adult wing size.
DUAL Control thus provides a powerful and flexible framework for identifying regulators of tissue regrowth and for comparing how different forms of injury—apoptotic versus necrotic—affect regenerative capacity.
See the projects page for details of these specific areas of research.